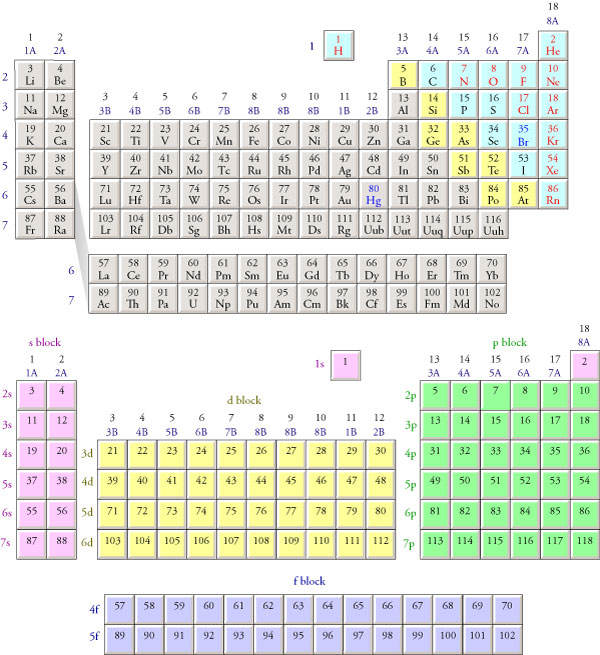
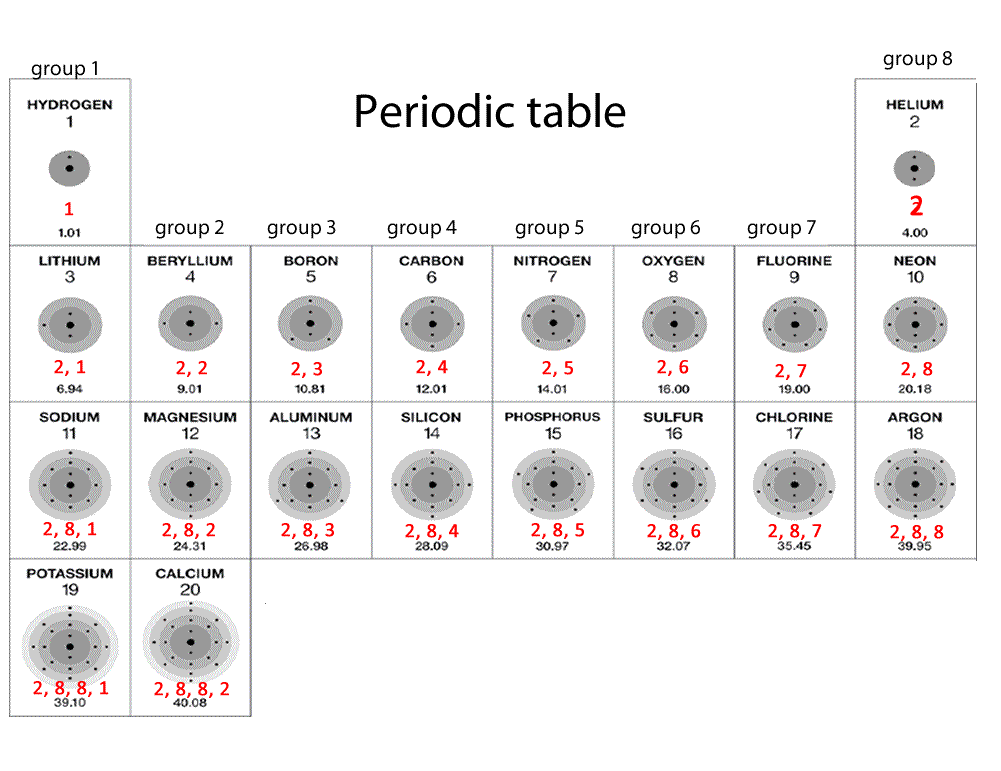
Electron Configuration Chart Periodic Table
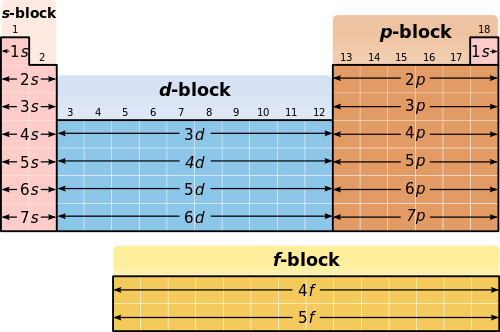
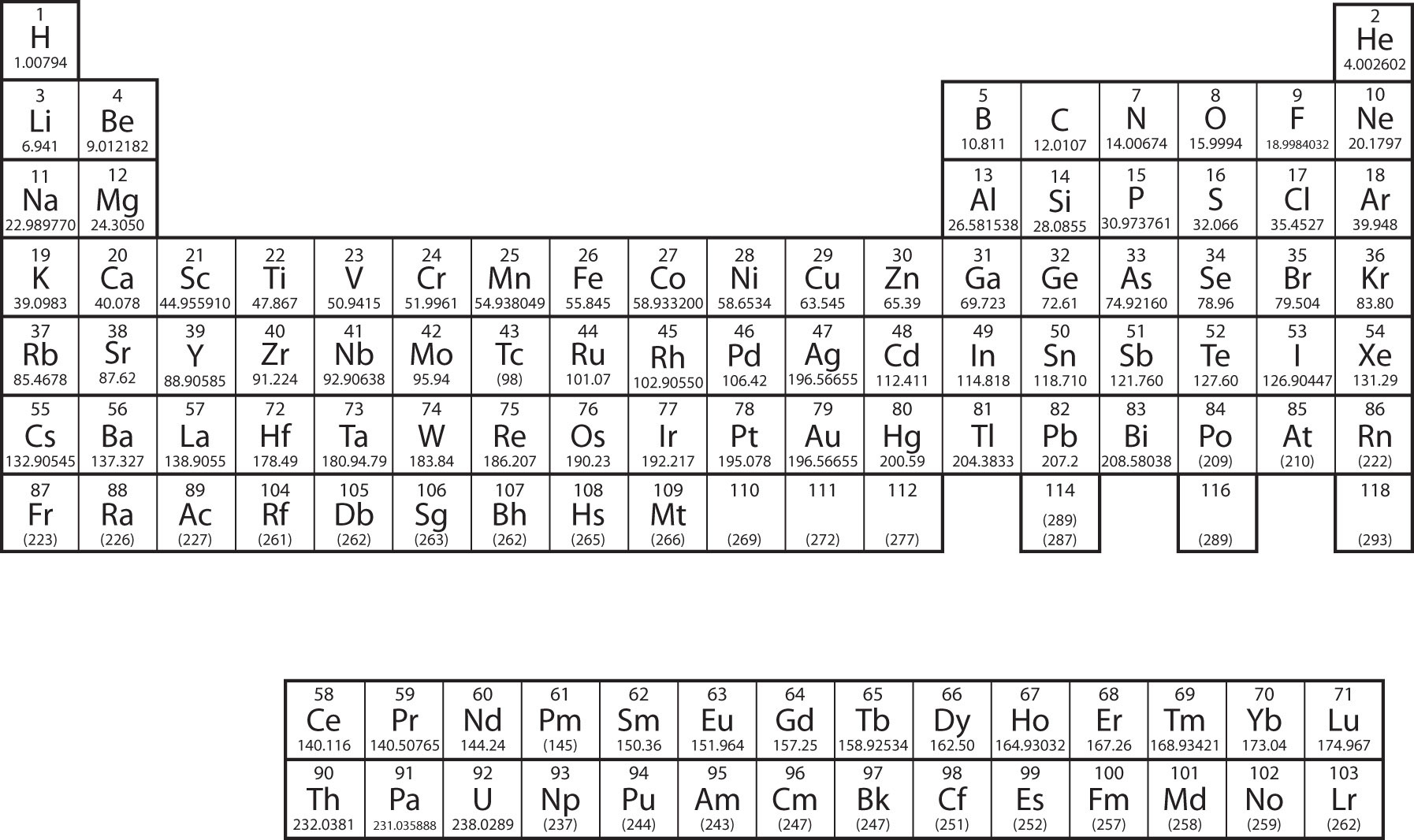
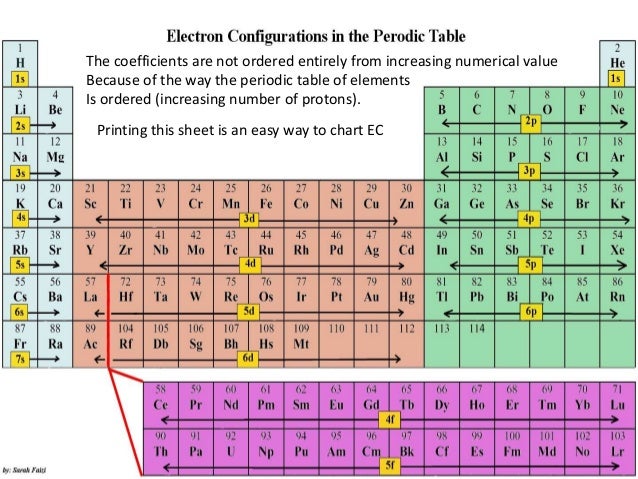
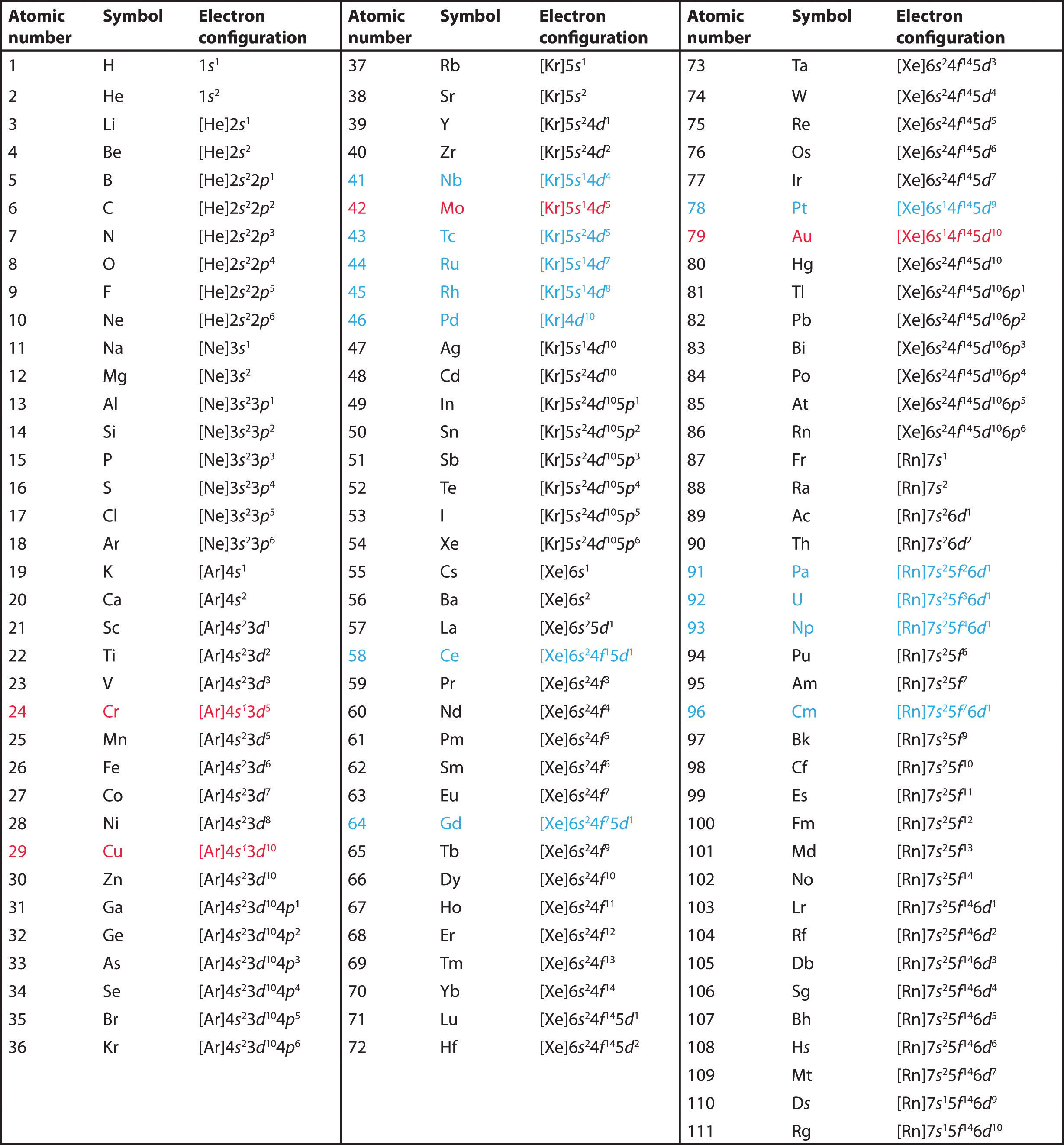
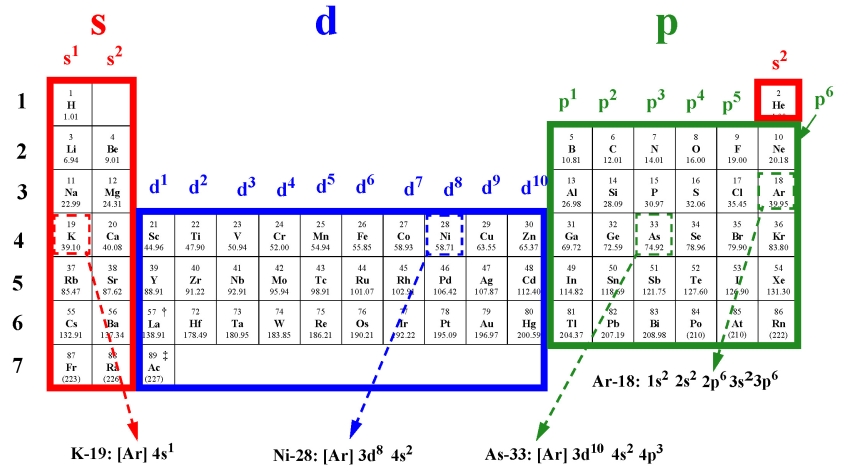
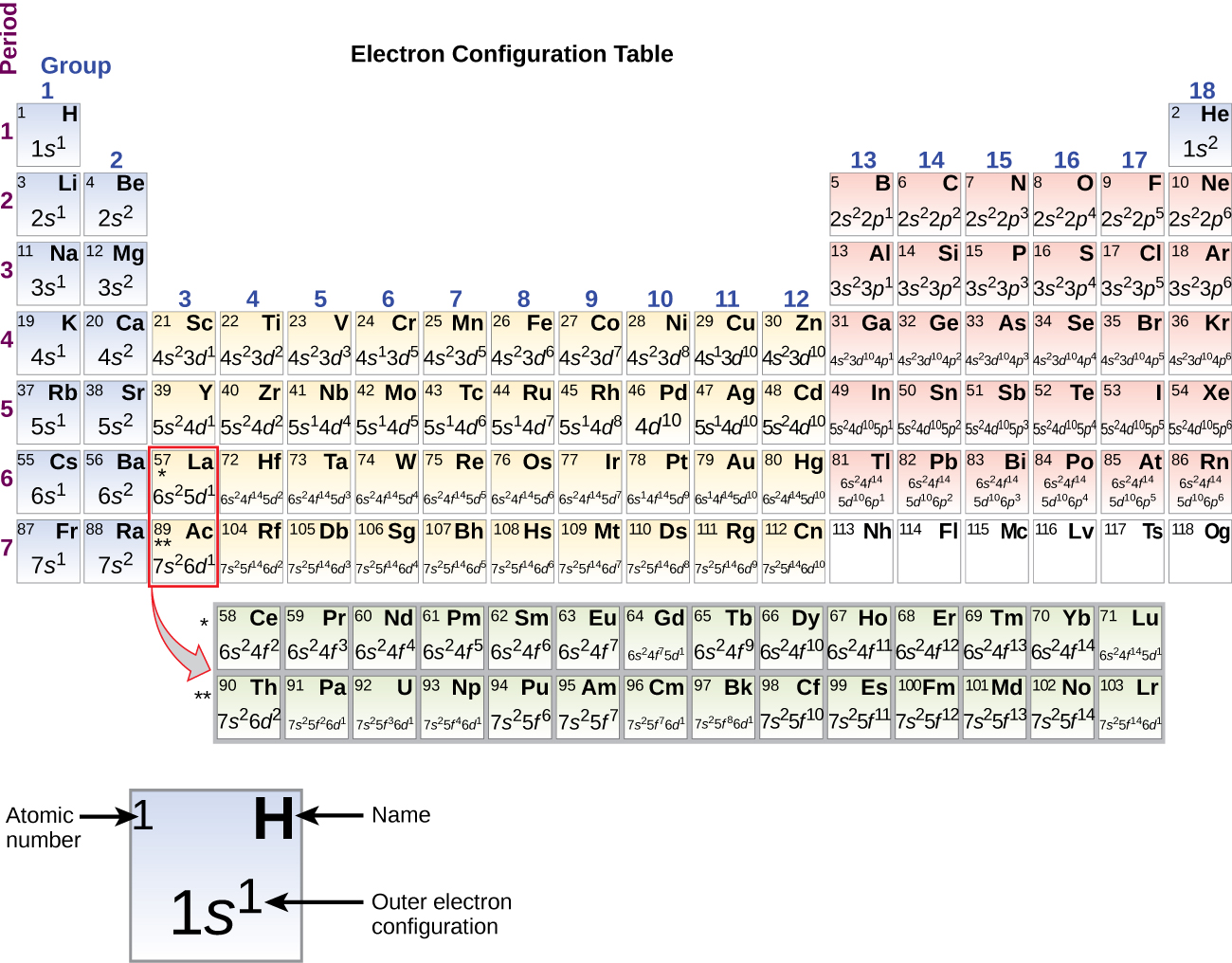
The electron configurations are written in the noble gas notation.
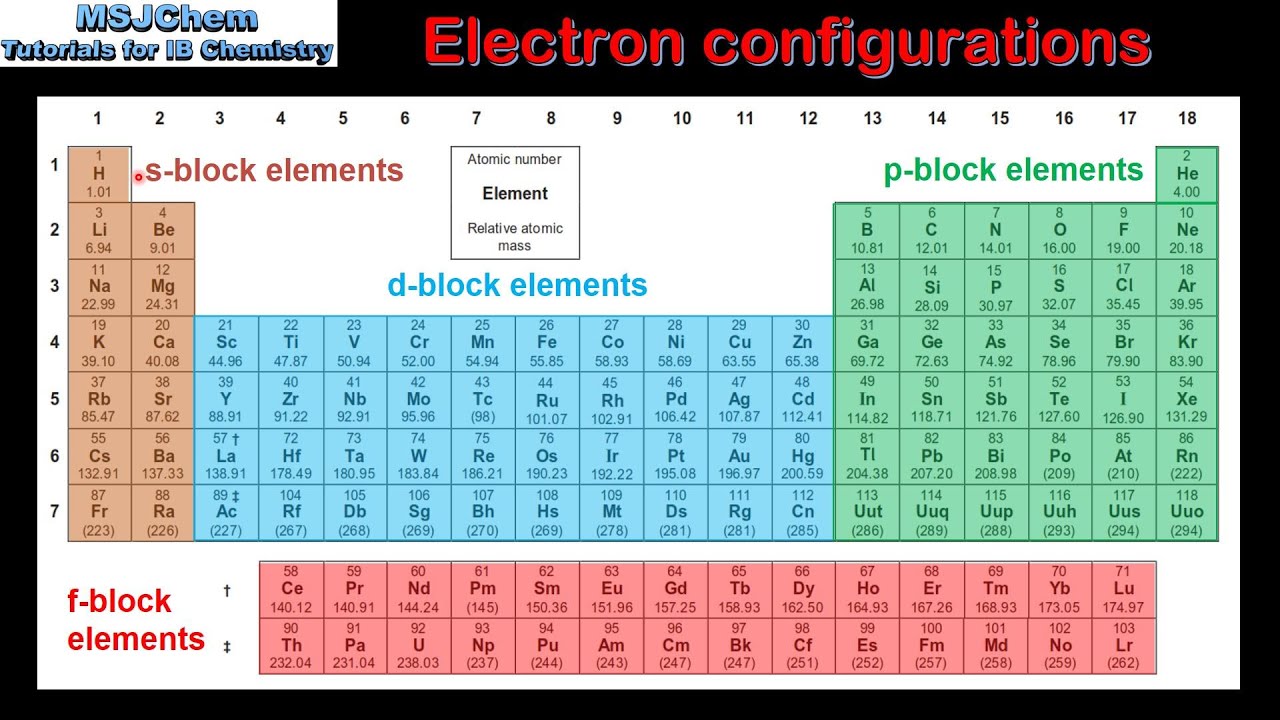
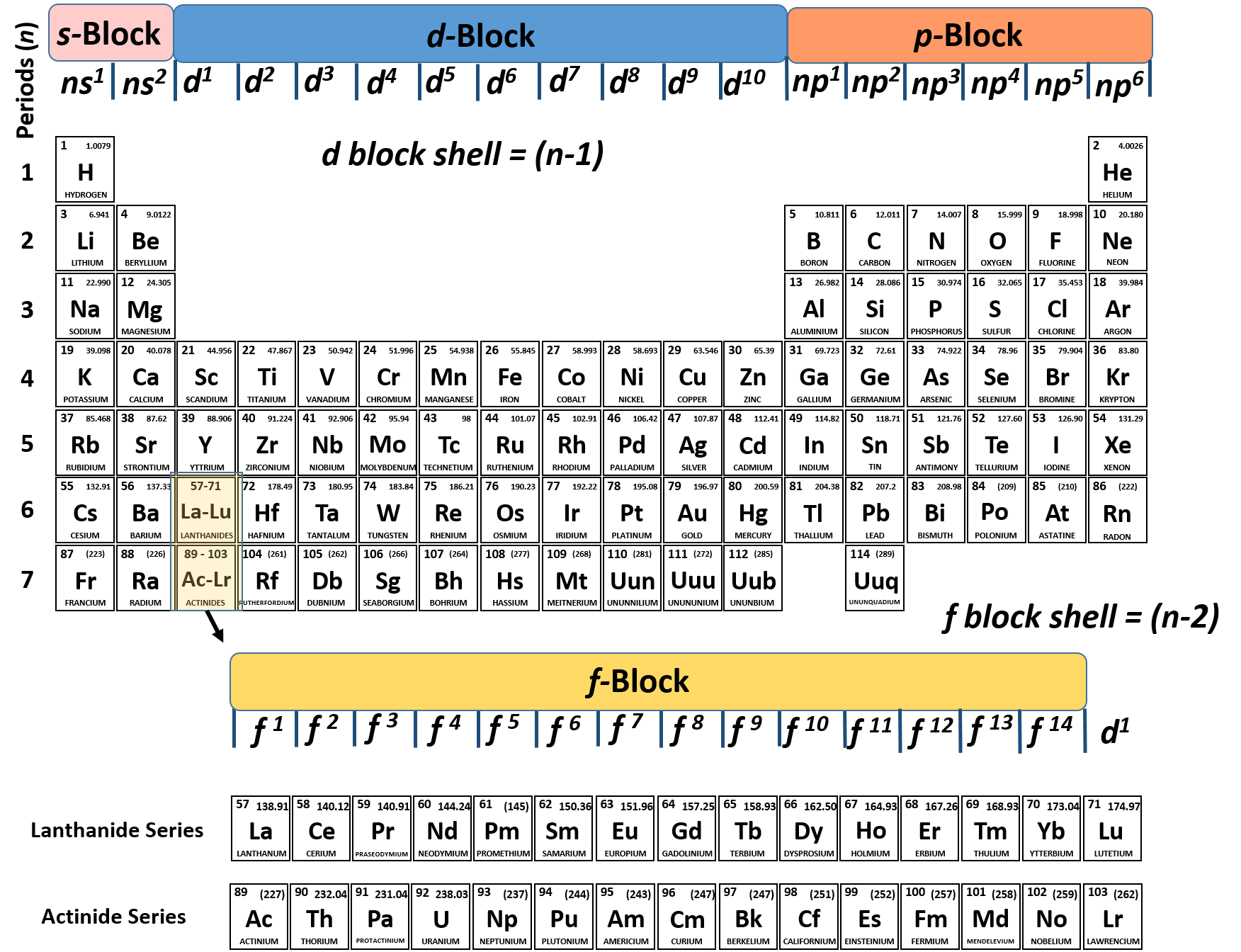
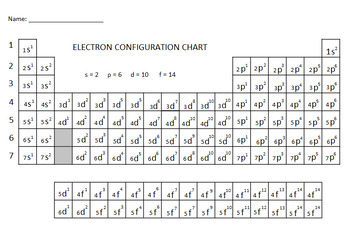
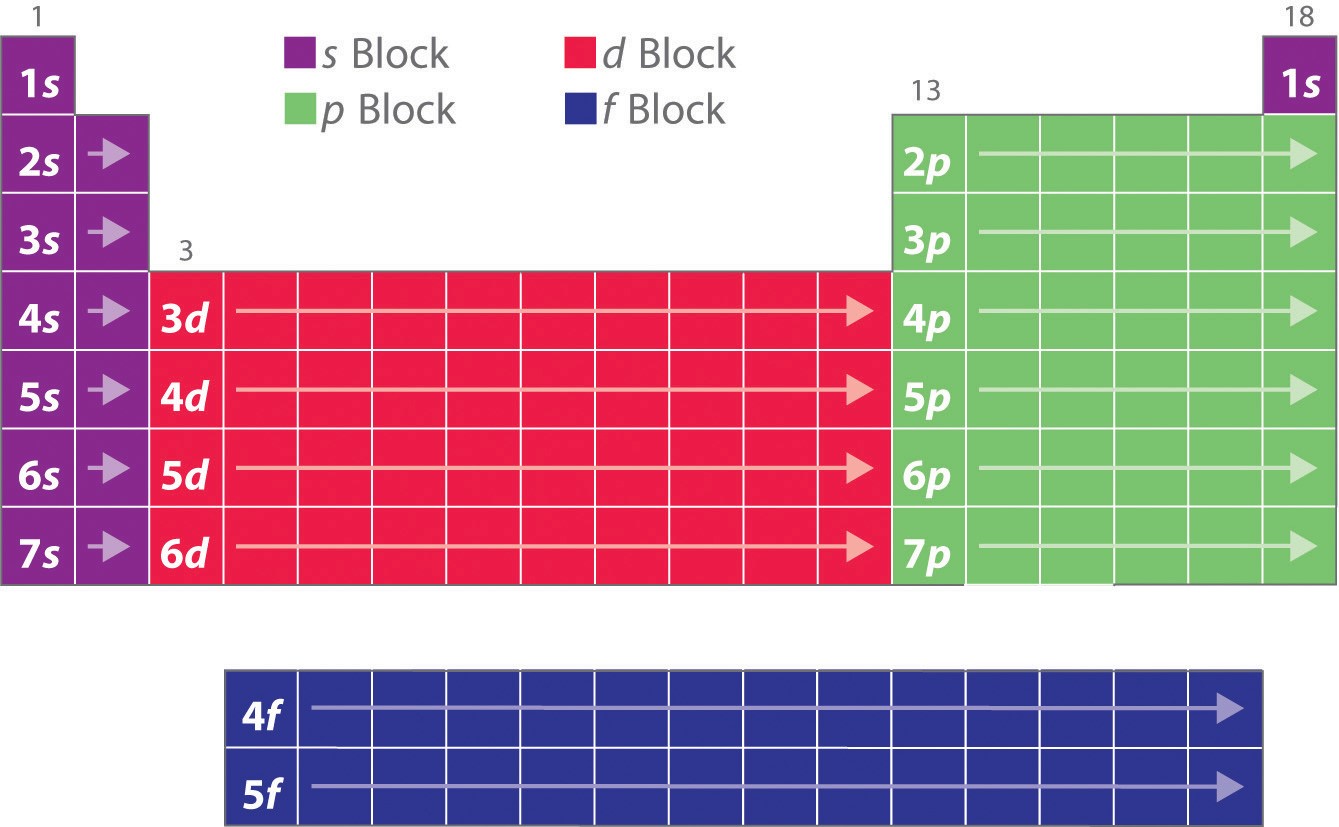
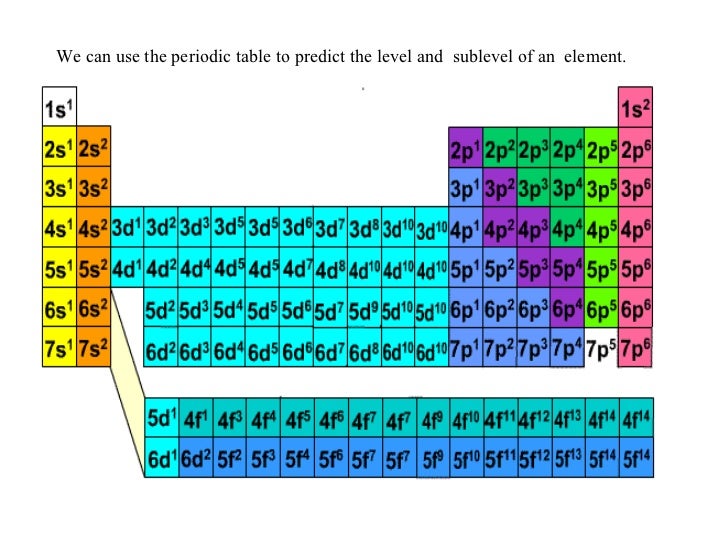
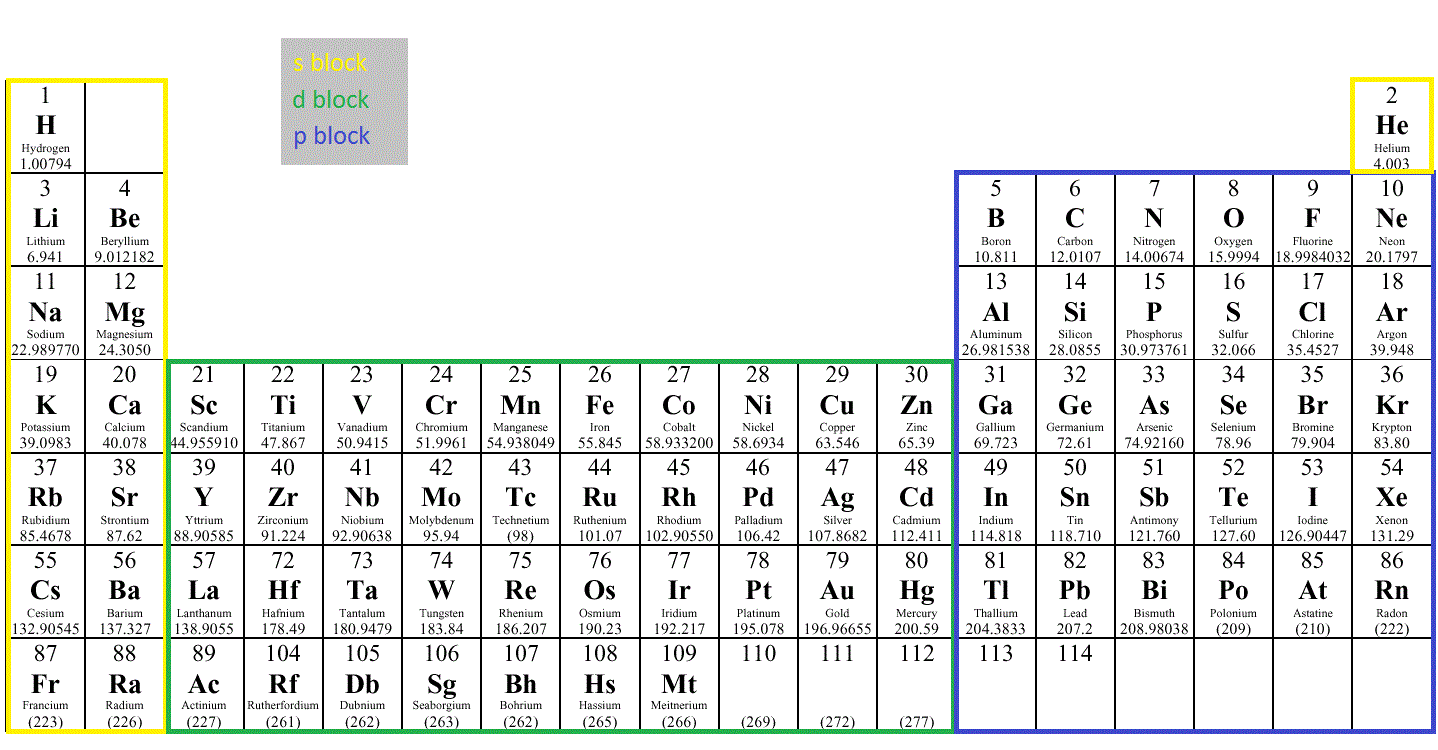
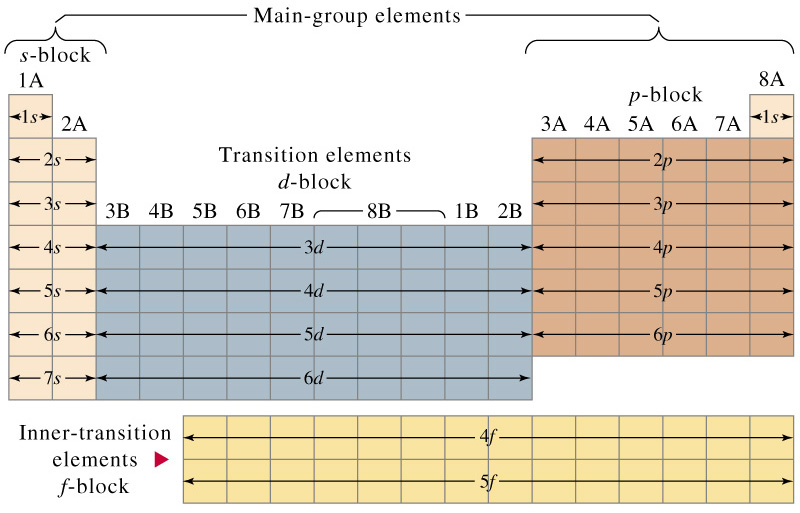
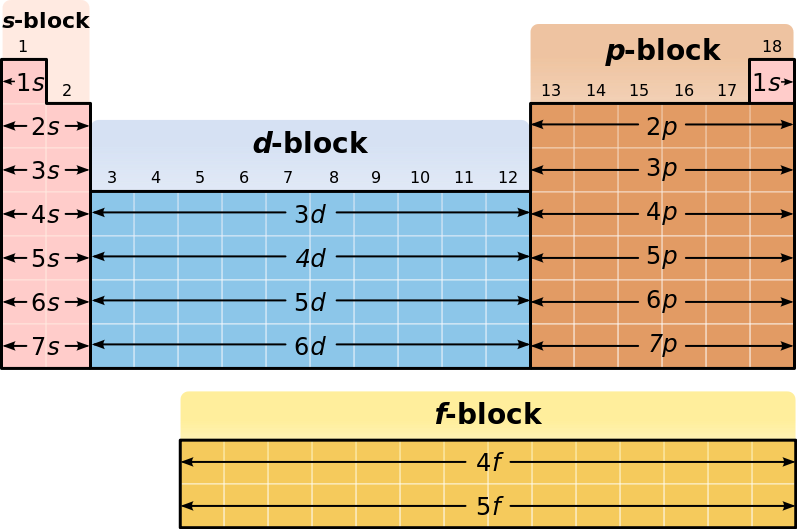
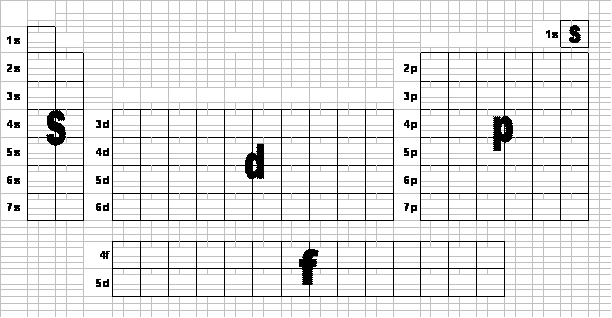
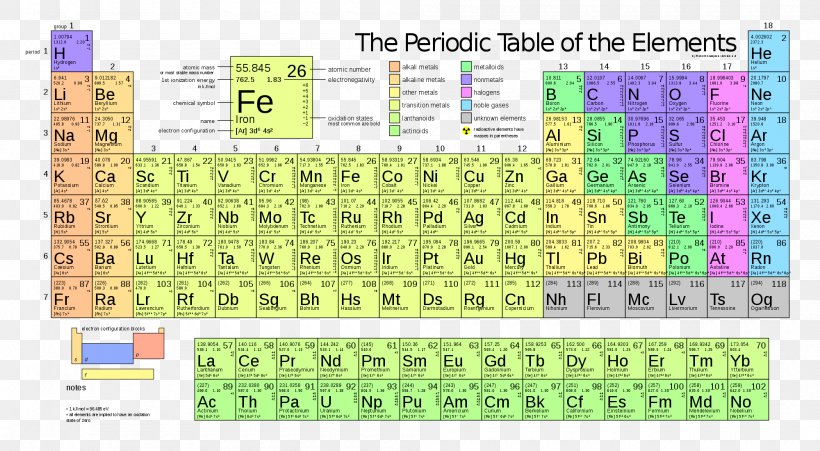
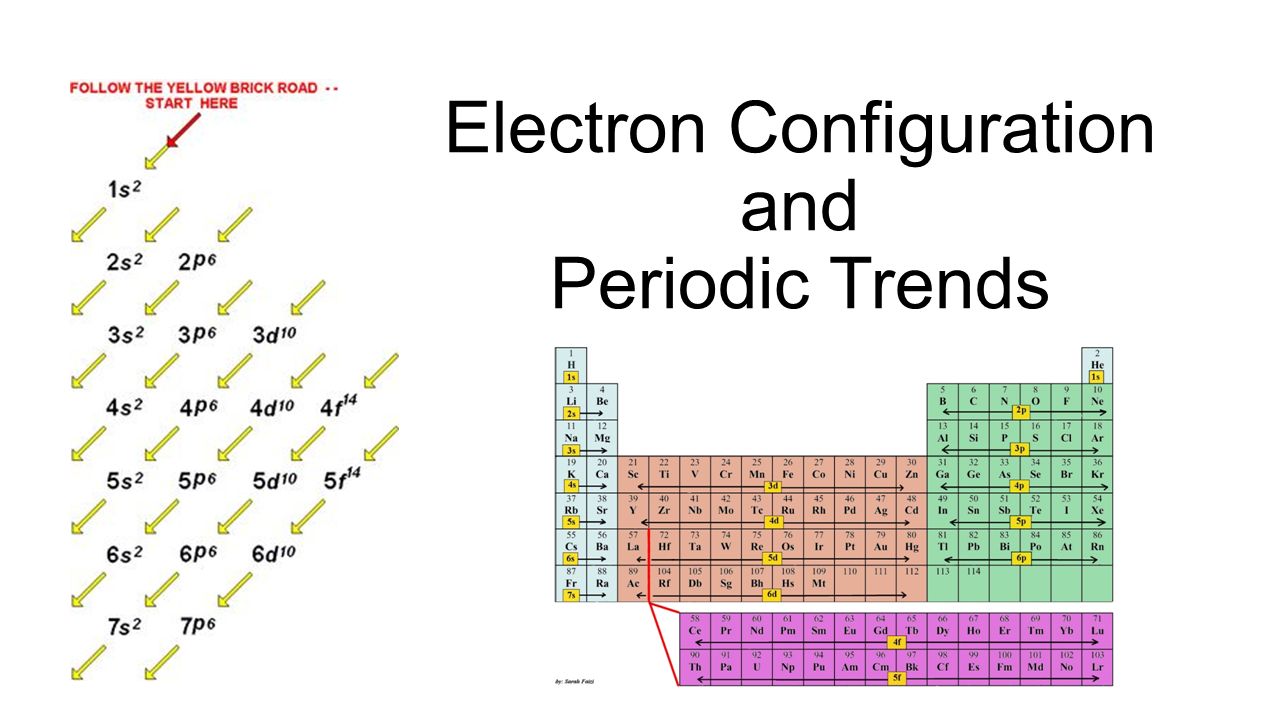
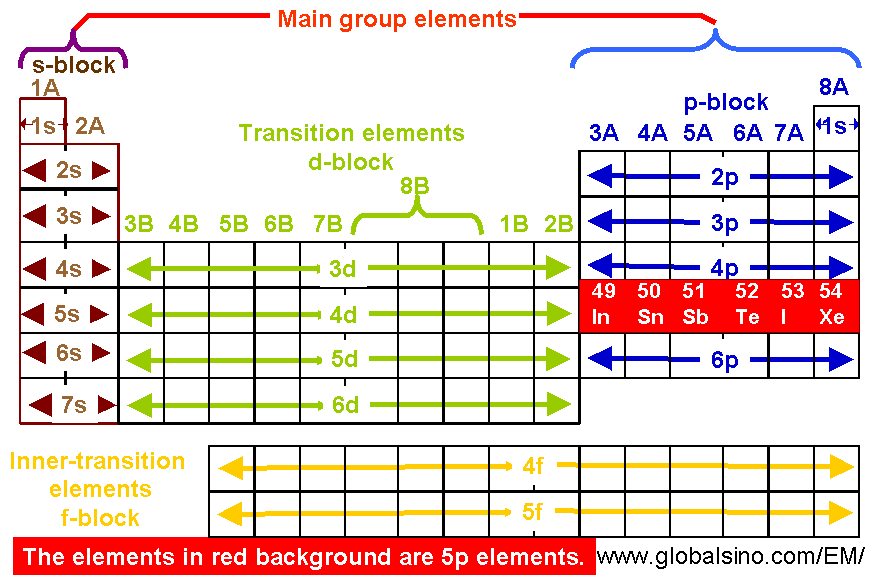
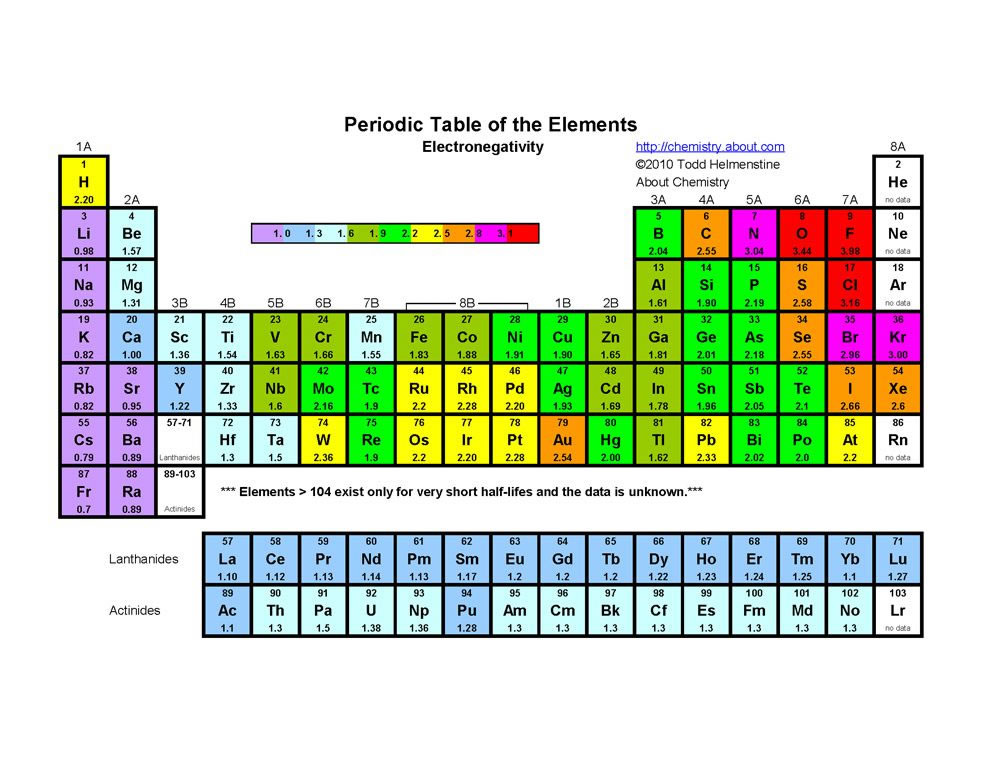
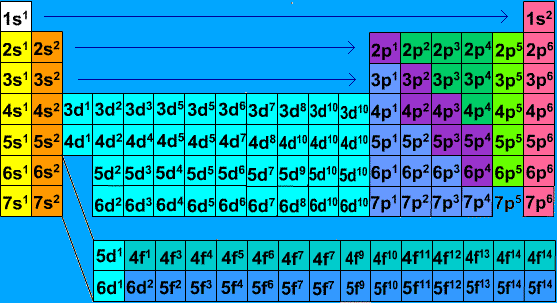
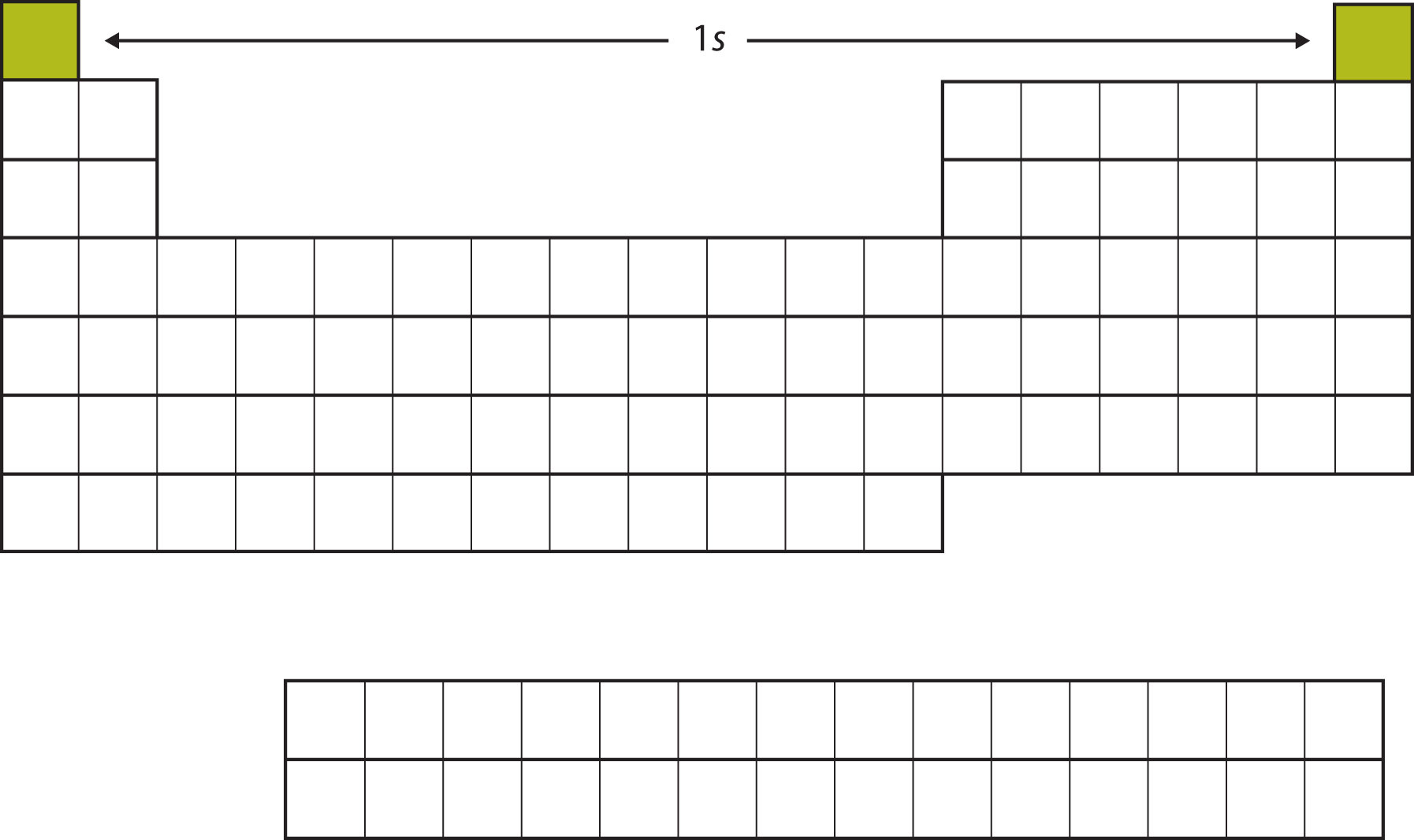
Electron configuration chart periodic table. The halogens f cl br etc are one electron short of a valence shell octet and are among the most reactive of the elements they are colored red in this periodic table. Electron configuration and the periodic table the electrons in an atom fill from the lowest to the highest orbitals. Because each orbital can have a maximum of 2 electrons there are 2 columns in the s block 6 columns in the p block 10 columns in the d block and 14 columns in the f block.
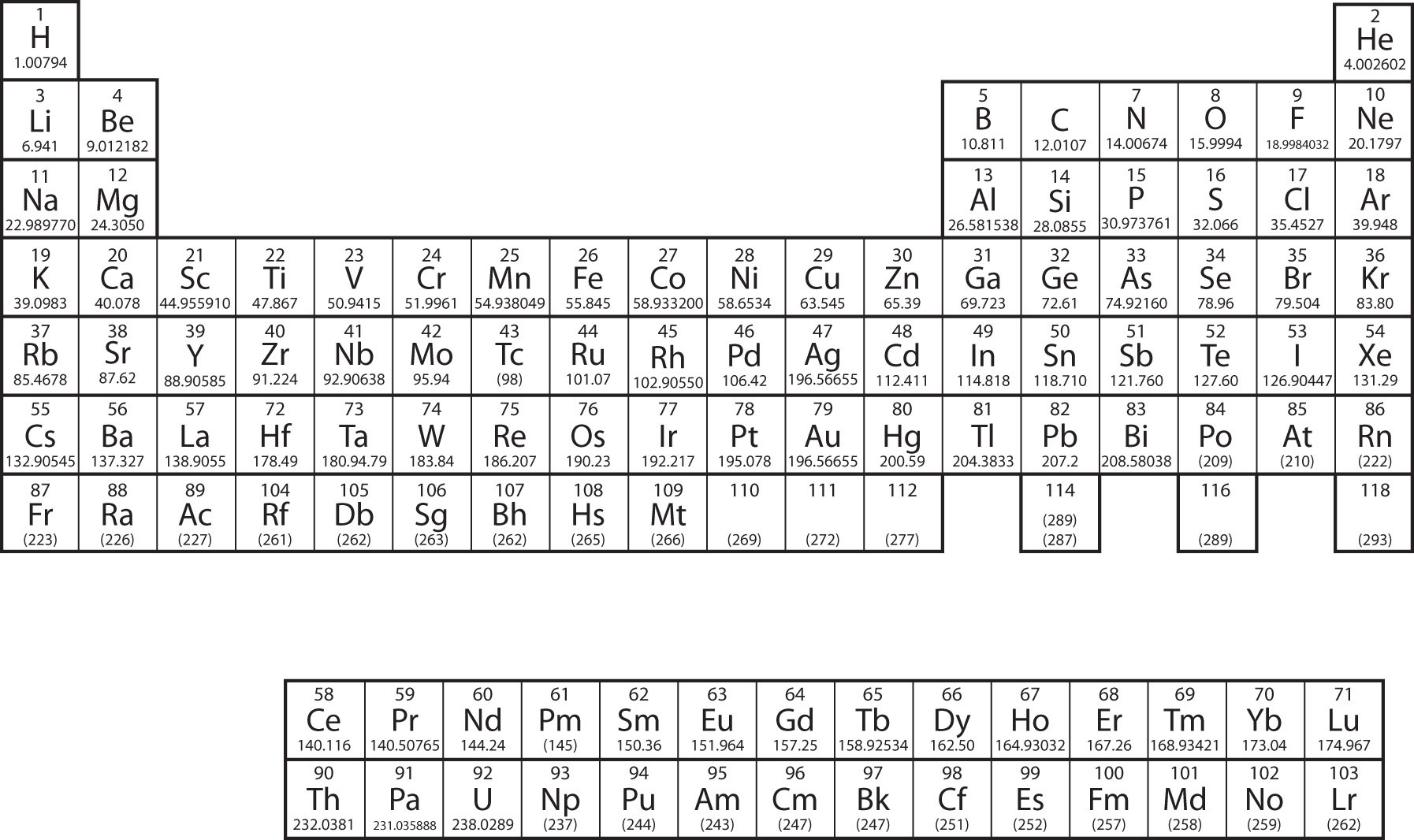
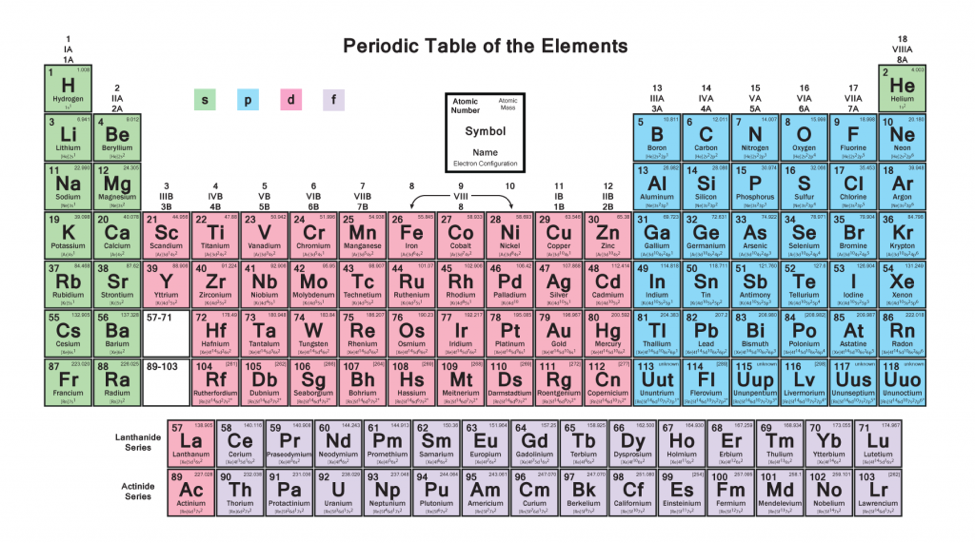
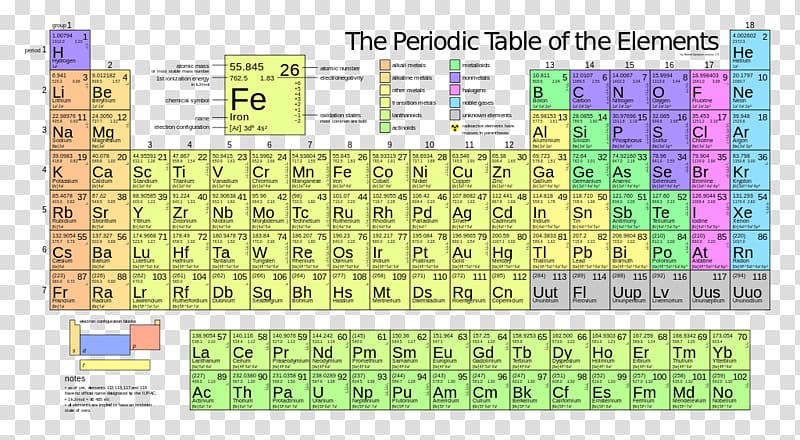
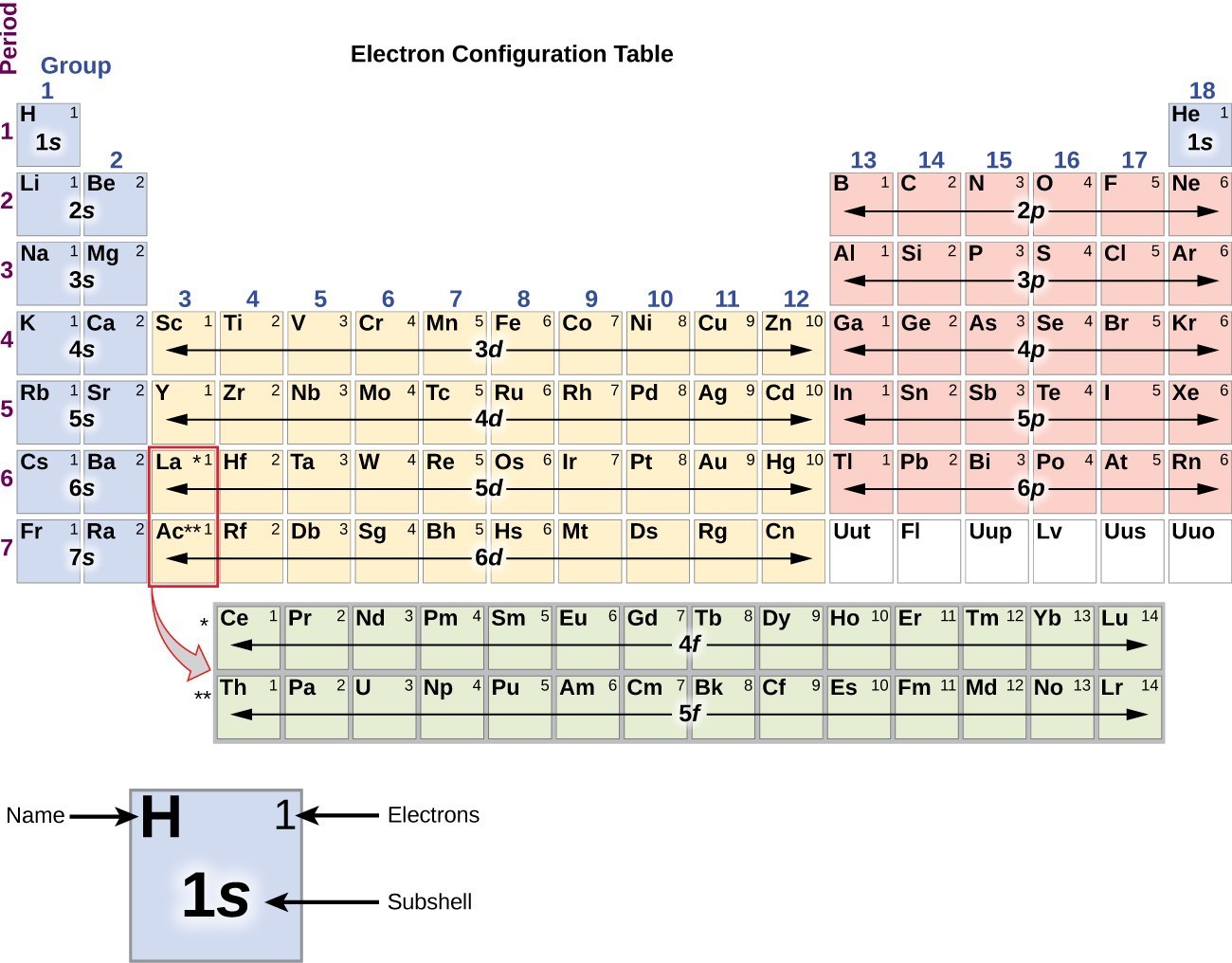
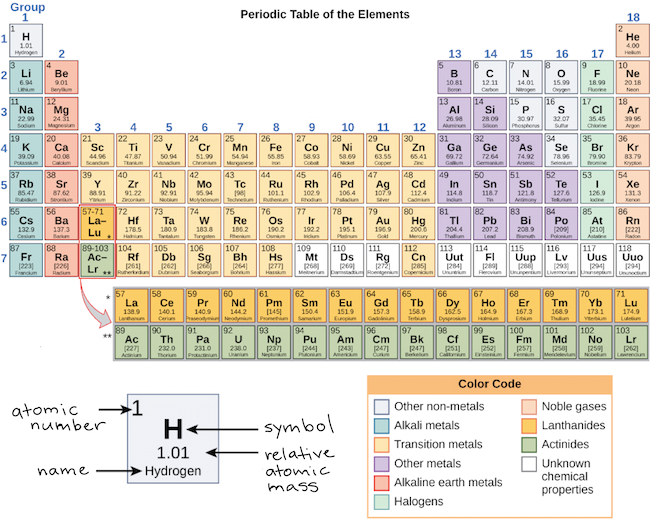
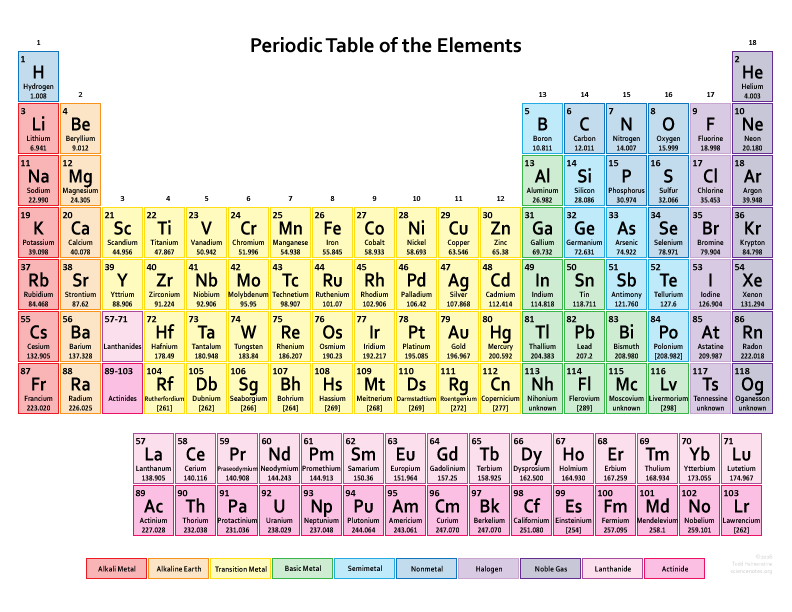
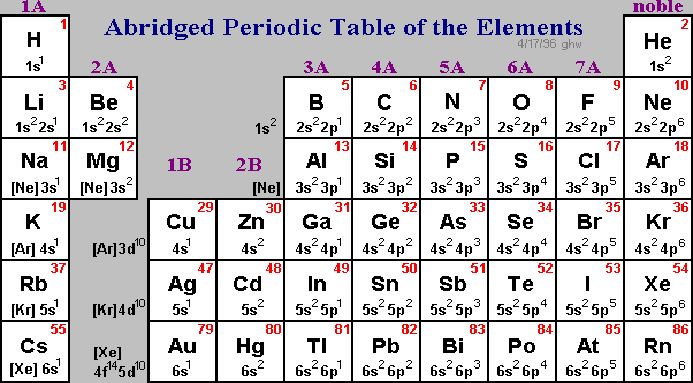
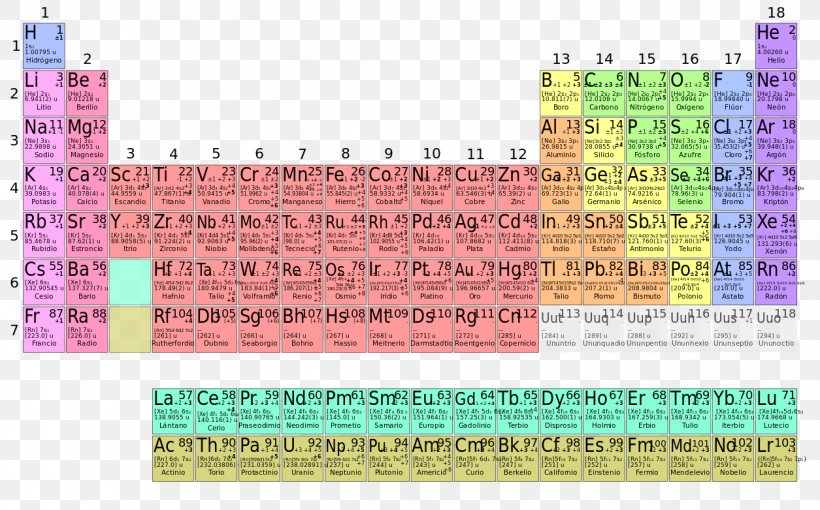
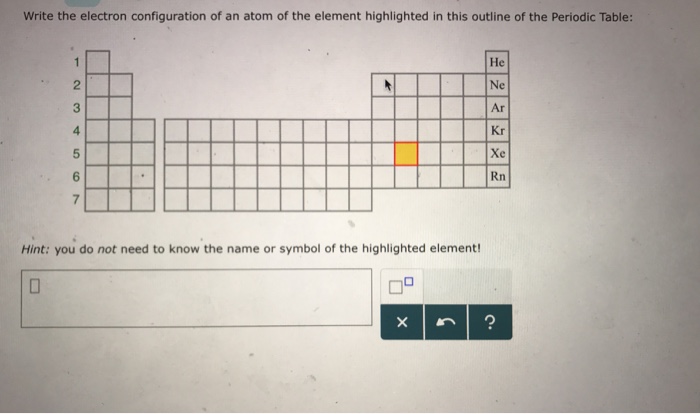
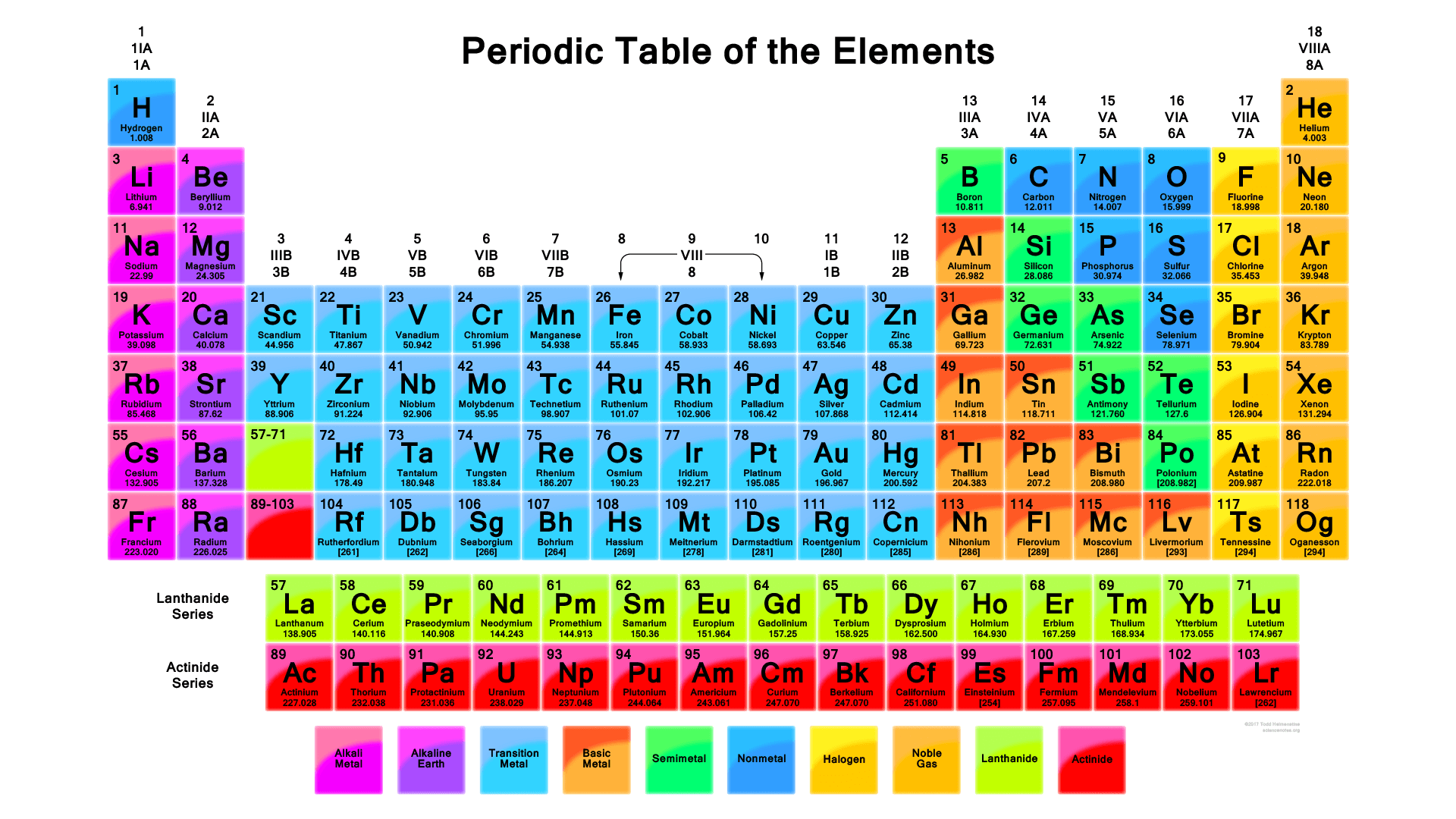
There are 118 elements in the periodic table. The electron configuration of an atom of any element is the of electrons per sublevel of the energy levels of an atom in its ground state. This periodic table contains each element s atomic number atomic mass symbol name and electron configuration.
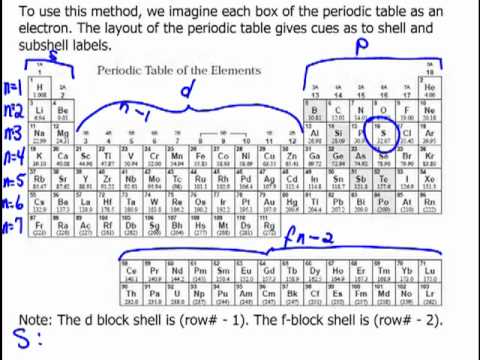
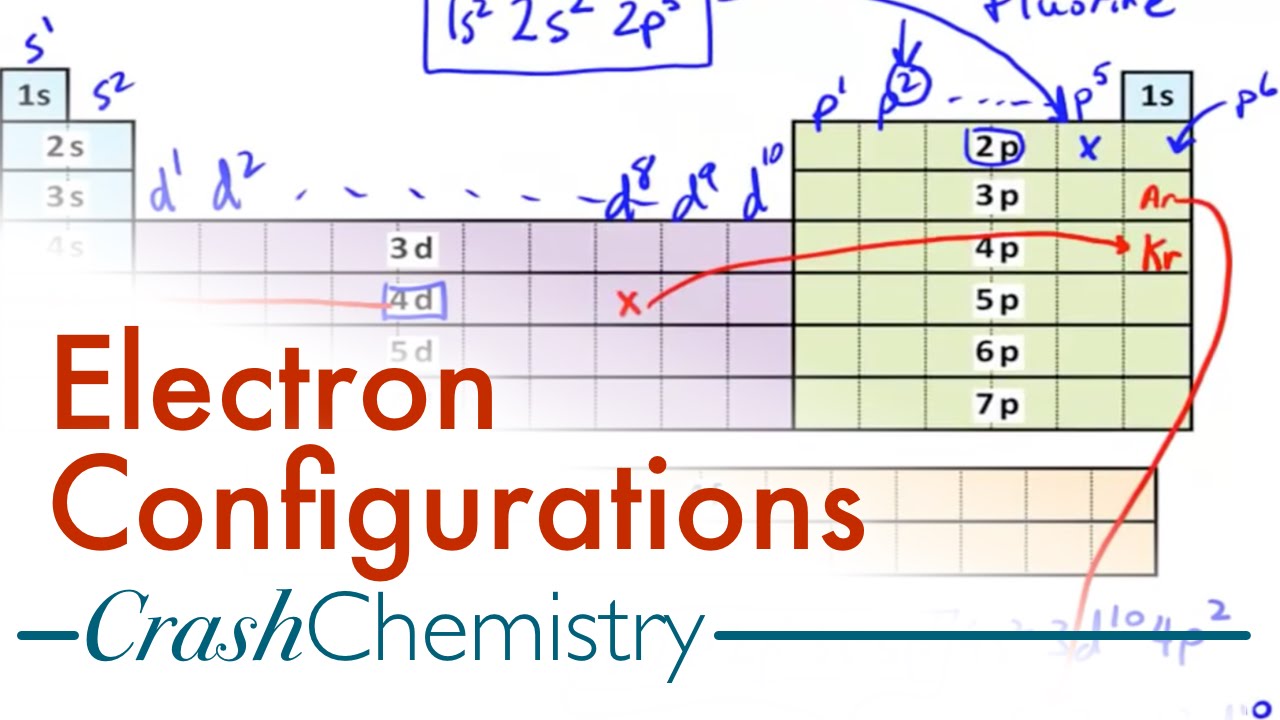
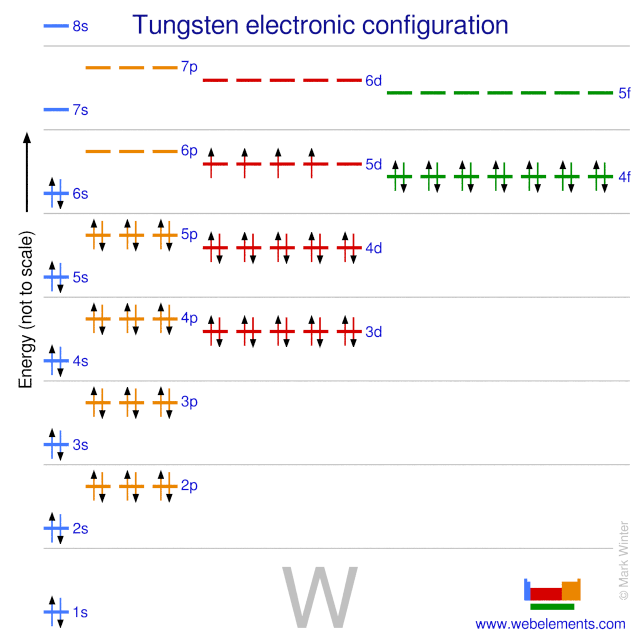
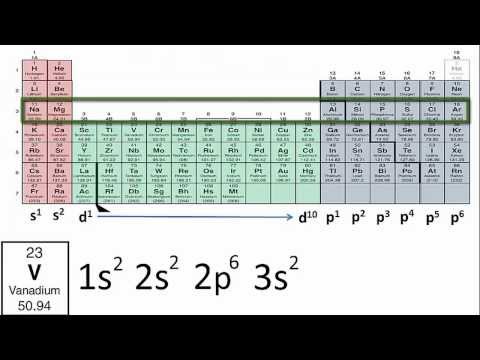
An electron configuration chart is a tabular representation of patterns in the electron configuration of elements as one goes down the periodic table of elements. The knowledge of the location of the orbitals on the periodic table can greatly help the writing of electron configurations for large atoms. The electron configurations of the elements are in figure 6 9 2.
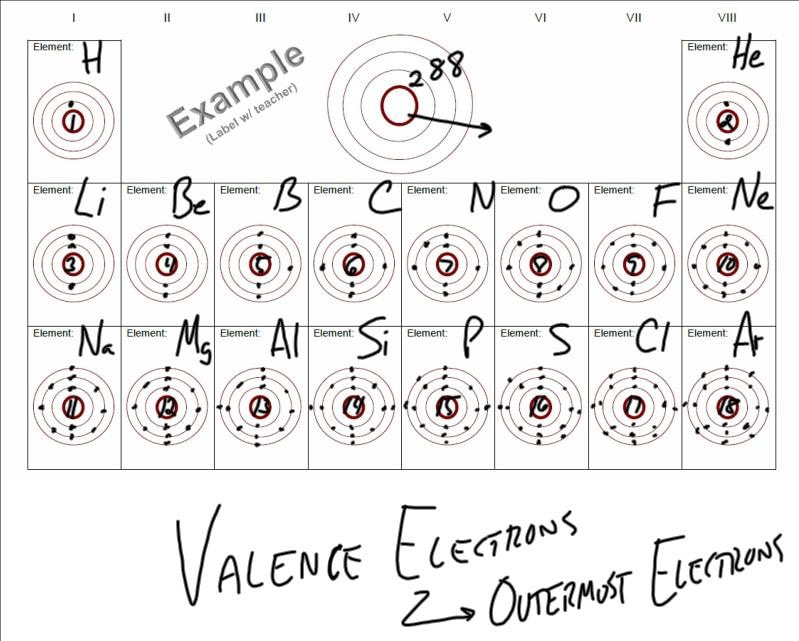
Each element has a unique atomic structure that is influenced by its electronic configuration which is the distribution of electrons across different orbitals of an atom. In the periodic table above these elements are colored beige. Hydrogen and helium are placed somewhat arbitrarily.
This article provides you with an electronic configuration chart for all these elements.



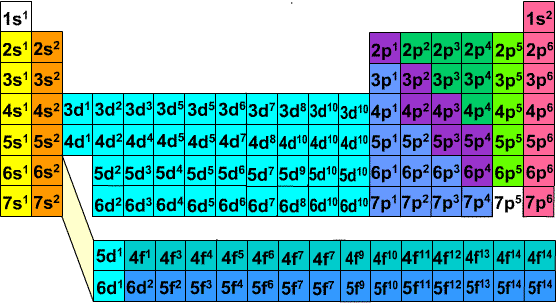




:max_bytes(150000):strip_icc()/ColorPeriodicTableEC-58b5c7fa3df78cdcd8bbb56f.png)






































.jpg?revision=1&size=bestfit&width=656&height=425)